"Dynamic acquisitions should be performed in IDH-mutant glioma patients to provide valuable information for the differential diagnosis of recurrence and treatment-related changes," noted Dr. Laura Rozenblum, a nuclear medicine physician at Pitié Salpêtrière Hospital in Paris, and colleagues in an article published on 11 by European Radiology.
The researchers analyzed the role of dynamic F-18 FDOPA-PET in differentiating progression and treatment-related changes in high-grade gliomas. They explained that the diagnostic accuracy of dynamic amino-acid PET, for distinguishing progression from treatment-related changes, is currently based on single-center nonhomogeneous glioma populations.
"Dynamic PET analysis is among the latest recommendations for PET imaging in neuro-oncology and has the advantage of being easily integrated into clinical practice," they stated.
Study logistics
The team retrospectively included patients with histologically confirmed high-grade glioma who underwent a dynamic F-18 FDOPA-PET between November 2015 and June 2020 in two French university hospitals (CHRU Nancy and Pitié-Salpêtrière).
Using four systems from three different vendors (PET/CTs Biograph 6, TruePoint, Siemens and Vereos, Philips Healthcare in Nancy; PET/CT Biograph mCT Flow, Siemens and 3-tesla PET/MR Signa GE Healthcare in Paris), PET was performed at least three months after the end of radiotherapy to reduce the risk of false positives. All patients had an MRI within the month prior to PET.
The group identified 106 patients with suspected glioma recurrences (WHO GIII, n = 38; GIV, n = 68; IDH-mutant, n = 35, IDH-wildtype, n = 71). Patients underwent dynamic F-18 FDOPA-PET/CT (n = 83) or PET/MRI (n = 23), and static tumor-to-background ratios (TBRs), metabolic tumor volumes, and dynamic parameters (time to peak and slope) were determined.
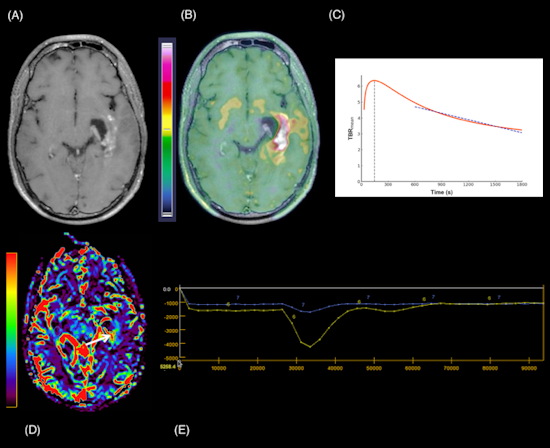
A 24-year-old man with a suspected GIII anaplastic astrocytoma IDH-mutant recurrence, referred for a PET/MRI. 3D Fat-saturated T1 with gadolinium sequence (A) shows enhanced lesions along the left temporal horn. Fused PET/MRI (B) highlights the high F-18 fluorodopa (FDOPA) uptake by the lesions (TBRmean = 2.3; TBRmax = 4.7; TSRmean = 1.2; TSRmax = 2.5; metabolic tumor volume = 29.71 cm3). The dynamic curve shows a washout pattern (slope = −5.42 h-1) (c, slope dark blue dotted line). Axial CBV reveals more voluminous areas (white arrow) that correspond to increased tumor angiogenesis (yellow line: lesion, blue line: contralateral normal brain parenchyma) (D, E). Follow-up MRI one month after the PET/MRI, confirms progression according to the Response Assessment in Neuro-Oncology (RANO) criteria. Figure courtesy of Dr. Laura Rozenblum et al, and European Radiology.
Surgery or the clinical-radiological six-month follow-up identified 71 progressions and 35 treatment-related changes. TBRmean, with a threshold of 1.8, best-differentiated glioma recurrence/progression from post-treatment changes in the whole population (sensitivity 82%, specificity 71%, p < 0.0001) whereas curve slope was only significantly different in IDH-mutant high-grade gliomas (HGGs, n = 25), the authors found.
In survival analyses, metabolic tumor volume was a clinical independent predictor of progression-free and overall survival on the multivariate analysis (p ≤ 0.01). A curve slope > -0.12/h was an independent predictor for longer progression-free survival in IDH-mutant HGGs, they added.
The final diagnosis was either defined by histopathology or a clinical-radiological follow-up at six months. Optimal F-18 FDOPA-PET parameter cutoffs were obtained by receiver-operating characteristic analysis. Predictive factors and clinical parameters were assessed using univariate and multivariate Cox regression survival analyses.
Rozenblum is also a research fellow at Massachusetts General Hospital in Boston. She gave special thanks to co-authors Prof. Antoine Verger, PhD, and Timothée Zaragori, PhD, both from Nancy, and Prof. Aurélie Kas, PhD, from Pitié Salpêtrière Hospital.
