F-18 DCFPyL (Pylarify) PET/MRI appears useful when determining whether men with low-risk prostate cancer are candidates for focal ablative therapy, according to research presented at RSNA 2022.
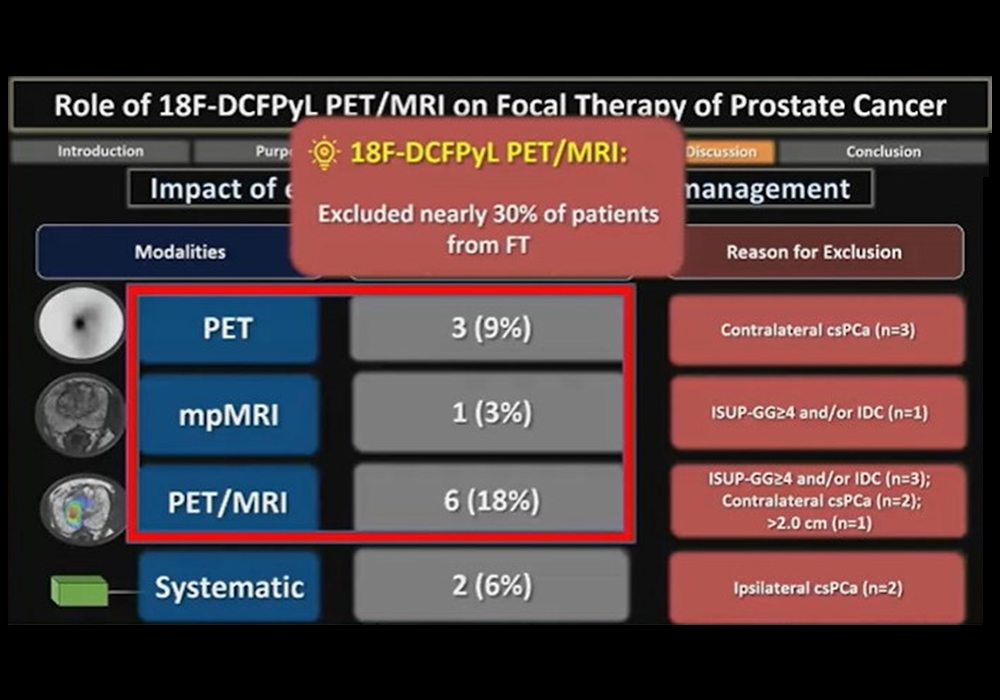
In a prospective trial, researchers at the University of Toronto sought to determine the role of Pylarify PET/MRI in the selection of patients with low or intermediate-risk prostate cancer for focal ablative therapy. They found the approach excluded nearly 30% of patients from the procedure, presenter Dr. Adriano Dias told attendees.
"Appropriate selection for focal ablative therapies is paramount in decreasing treatment failure," he said.
Focal ablative therapy in prostate cancer patients is a treatment that entails the use of extreme temperatures to destroy tissue. In this treatment, the area of the prostate that contains the lesion is targeted, rather than treating the entire prostate gland. Imaging plays a key role by defining the diseased area and helps determine whether the approach may be successful or whether another approach is warranted.
Pylarify is a PET imaging radiotracer approved by the U.S. Food and Drug Administration for imaging in men with late-stage prostate cancer that has returned after radical prostatectomy. Few studies have tested the tracer in patients with early disease, Dias said.
In this study, the group explored the use of Pylarify PET/MRI imaging for localizing the target area for ablative therapy. They enrolled 34 men with biopsy-proven low or intermediate-risk cancer who were potential candidates for focal ablative therapy based on initial biopsies.
All participants underwent Pylarify PET/MRI between June 2017 and October 2019, with diagnostic performances calculated and compared among PET, multiparametric (mp) MRI, and PET/MRI. Receiver operating characteristic (ROC) curves were drawn for each modality, and their area under the curve (AUC) was calculated.
Dr. Adriano Dias highlighted results of a trial using Pylarify radiotracer in selecting patients for focal ablative therapy. Image courtesy of Adriano Dias.
Forty out of 67 (60%) lesions identified in patients on PET, mpMRI, or PET/MRI were malignant, and 34 of these 40 (85%) were clinically significant, according to the findings. On a lesion-level analysis in these, the sensitivity of PET was higher than of mpMRI and PET/MRI (91% vs. 76% and 79%), but it showed a lower specificity (39% vs. 85% and 88%, p < 0.001). The calculated AUCs were 0.65 for PET, 0.81 mpMRI, and 0.84 for PET/MRI.
Ultimately, 22 patients were excluded from focal ablative therapy. PET detected contralateral clinically significant prostate cancer in 9% of patients, while mpMRI detected more advanced disease in 3%, and PET/MRI detected more advanced disease in 11% and contralateral prostate cancer in 6% of patients.
"Overall, F-18 DCFPyL PET/MRI (PET, mpMRI and PET/MRI) excluded nearly 30% of patients from focal therapy," Dias noted.
Limits of the study included the small number of patients and potential selection bias, given that all men referred to the study had prior positive biopsies for clinically significant prostate cancer, Dias said.
"Larger prospective studies assessing incorporation of PSMA PET/MRI in this select patient population are needed to determine whether this approach improves patient outcomes," he concluded.
