A first-in-human evaluation of the novel theranostic pair 68Ga-DOTA-5G / 177Lu-DOTA-ABM-5G has confirmed its ability to successfully detect metastatic pancreatic cancer and visualize targeted treatment of the disease. According to research presented at the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, the pair was well tolerated among patients and has the potential to make a significant improvement in the clinical care and treatment of patients with metastatic pancreatic cancer, as well as other cancers. A grouping of 68Ga-DOTA-5G and 177Lu-DOTA-ABM-5G images has been selected as the 2023 SNMMI Henry N. Wagner, Jr., Image of the Year.
Each year, SNMMI chooses an image that best exemplifies the most promising advances in the field of nuclear medicine and molecular imaging. The state-of-the-art technologies captured in these images demonstrate the capacity to improve patient care by detecting disease, aiding diagnosis, improving clinical confidence and providing a means of selecting appropriate treatments. This year, the SNMMI Image of the Year was chosen from more than 1,500 abstracts submitted for the meeting.
Despite exhaustive testing and some encouraging advances in first- and second-line treatments, pancreatic cancer remains the most lethal type of cancer. The American Cancer Society estimates that more than 64,000 people will be diagnosed with pancreatic cancer in 2023, and more than 50,000 of them will die from the disease—a 5-year survival rate of only 12%.
“Poor patient outcomes are due partly to the late stage at which the majority of patients are diagnosed and partly to the limited and relatively ineffective treatment options,” stated Julie L. Sutcliffe, PhD, professor of internal medicine and biomedical engineering, director of the Cyclotron and Radiochemistry Facility in the Center for Molecular and Genomic Imaging and co-director of the Center for Molecular and Genomic Imaging at the University of California–Davis. “Our goal with this study was to develop a theranostic strategy to facilitate both earlier detection and more effective treatment for this devastating malignancy.”
The integrin αvβ6 is an epithelial-specific cell surface receptor that is undetectable in healthy adult epithelial cells but is significantly up-regulated in a wide range of epithelial cell-derived cancers, including pancreatic cancer, which makes it a very attractive clinical target for detection and targeted treatment. In the study, researchers in Sutcliffe’s laboratory radiolabeled αvβ6-integrin-targeted peptides to create the theranostic pair 68Ga-DOTA-5G / 177Lu-DOTA-ABM-5G, which was evaluated for its safety and effectiveness.
Patients with metastatic or locally advanced pancreatic cancer who demonstrated advanced disease that did not respond to at least one prior standard-of-care treatment were recruited to the study. Patients underwent 68Ga-DOTA-5G PET/CT scans, then vital signs, blood samples and EKG were checked one and seven days after imaging. Patients with lesions with an SUVmax more than two times the normal lung or liver uptake were eligible for 177Lu-DOTA-ABM-5G treatment. Eligible patients received a single treatment with 177Lu-DOTA-ABM-5G and underwent whole body planar imaging and SPECT/CT imaging at approximately one and seven days following administration for evaluation of biodistribution and dosimetry. Vital signs, blood samples and EKG were checked one, seven and 14 days after the treatment.
To date, 17 patients have been imaged with 68Ga-DOTA-5G PET/CT and 14 patients have been treated with 177Lu-DOTA-ABM-5G. Both the 68Ga-DOTA-5G and 177Lu-DOTA-ABM-5G agents were well tolerated, and no drug-related serious adverse events were observed. 68Ga-DOTA-5G PET/CT imaging was able to detect bone, lung and hepatic metastasis, and the 177Lu-DOTA-ABM-5G was taken up and retained by the lesions detected by 68Ga-DOTA-5G PET/CT imaging. The average absorbed doses to kidneys and bone marrow (respectively) obtained to date are 3.7 and 0.01 Gy for dose level 1 (25mCi), 6.93 and 0.02 Gy for dose level 2 (50 mCi), 10.03 and 0.03 Gy for dose level 3 (100 mCi), and 14.56 and 0.04 Gy for dose level 4 (150 mCi).
“Results from this Phase I trial will pave the way for additional clinical trials in patients with other malignancies including, but not limited to, non-small cell lung cancer, breast cancer, and head and neck cancer,” Sutcliffe said. “The promising pharmacokinetic profile of the 177LuDOTA-ABM-5G therapy suggests that off-target adverse events are not expected, posing a major improvement over current treatments, which are known to cause bone marrow toxicity, hepatobiliary toxicity and cardiotoxicity. This approach has the potential to significantly improve the clinical care and treatment for patients with metastatic disease.”
“Findings from this study will add to the rapidly advancing landscape of theranostics,” noted SNMMI Scientific Program chair Heather Jacene, MD. “This new theranostic approach to detecting and treating pancreatic cancer, as shown in the Image of the Year, is a prime example of how personalized medicine can noninvasively detect disease, appropriately select and effectively treat patients, and have a significant, positive impact on the lives of many.”
The Phase I arm of this study will be completed in the summer of 2023, and researchers anticipate that a second trial in patients with metastatic non-small cell lung cancer will open to enrollment in fall 2023. A third trial for patients with metastatic carcinoma of any primary origin is expected to commence in the winter of 2023.
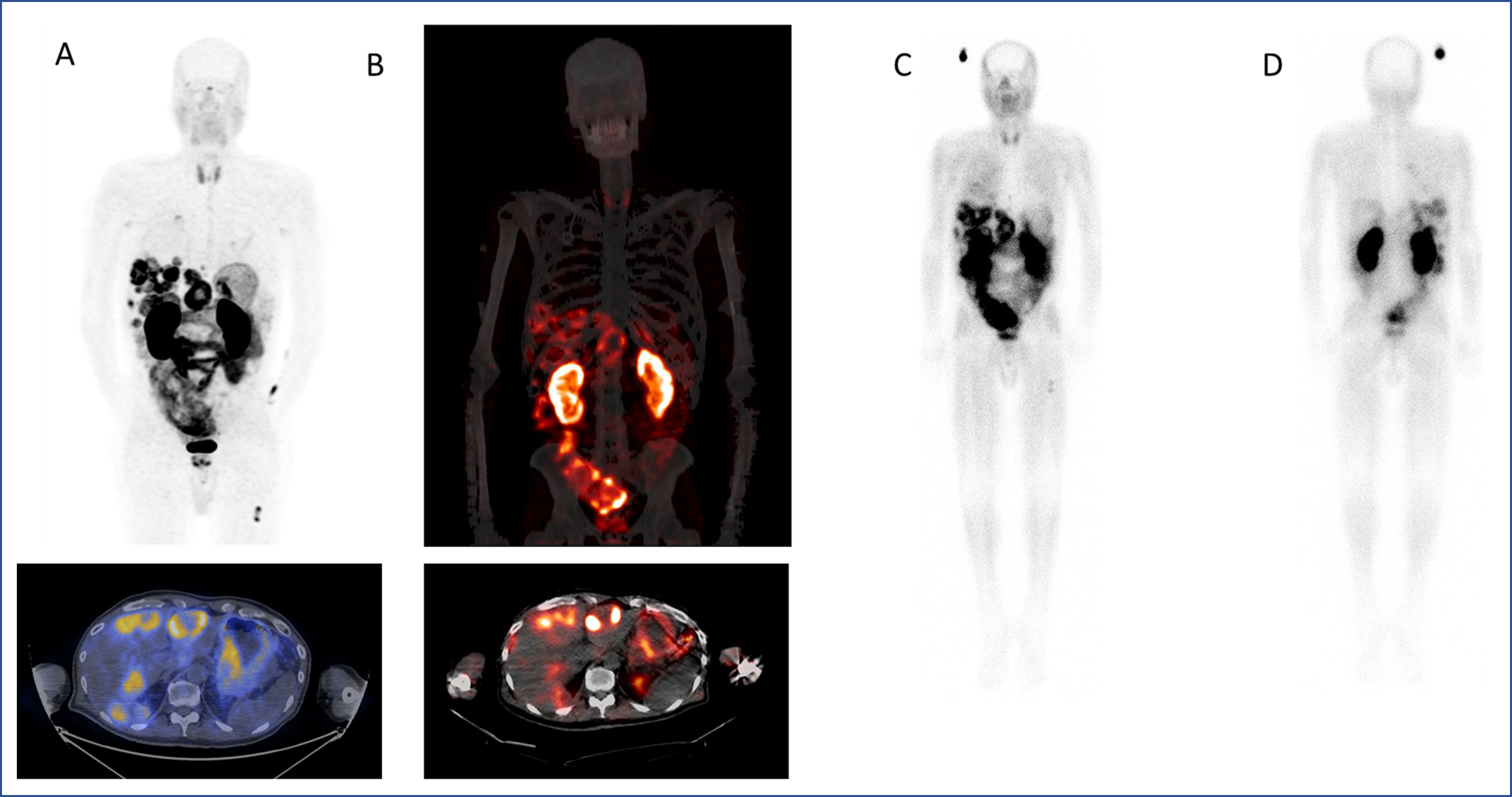
Figure 1. A. 68Ga-DOTA-5G PET/CT images (one hour postinjection). B. 177Lu-DOTA-ABM-5G SPECT/CT images (one day post injection). C. 177Lu-DOTA-ABM-5G whole body planar image (anterior, one day post injection). D. 177Lu-DOTA-ABM-5G whole body planar image (posterior, one day post injection).
Abstract 1300. “First-in-human study of the theranostic pair [68Ga]Ga DOTA-5G and [177Lu]Lu DOTA-ABM-5G in pancreatic adenocarcinoma.” Ryan Davis, Cameron Foster, Tanushree Ganguly, Sven Hausner, Edward Kim, Emilie Roncali, and Julie Sutcliffe, University of California, Davis, Davis, California.