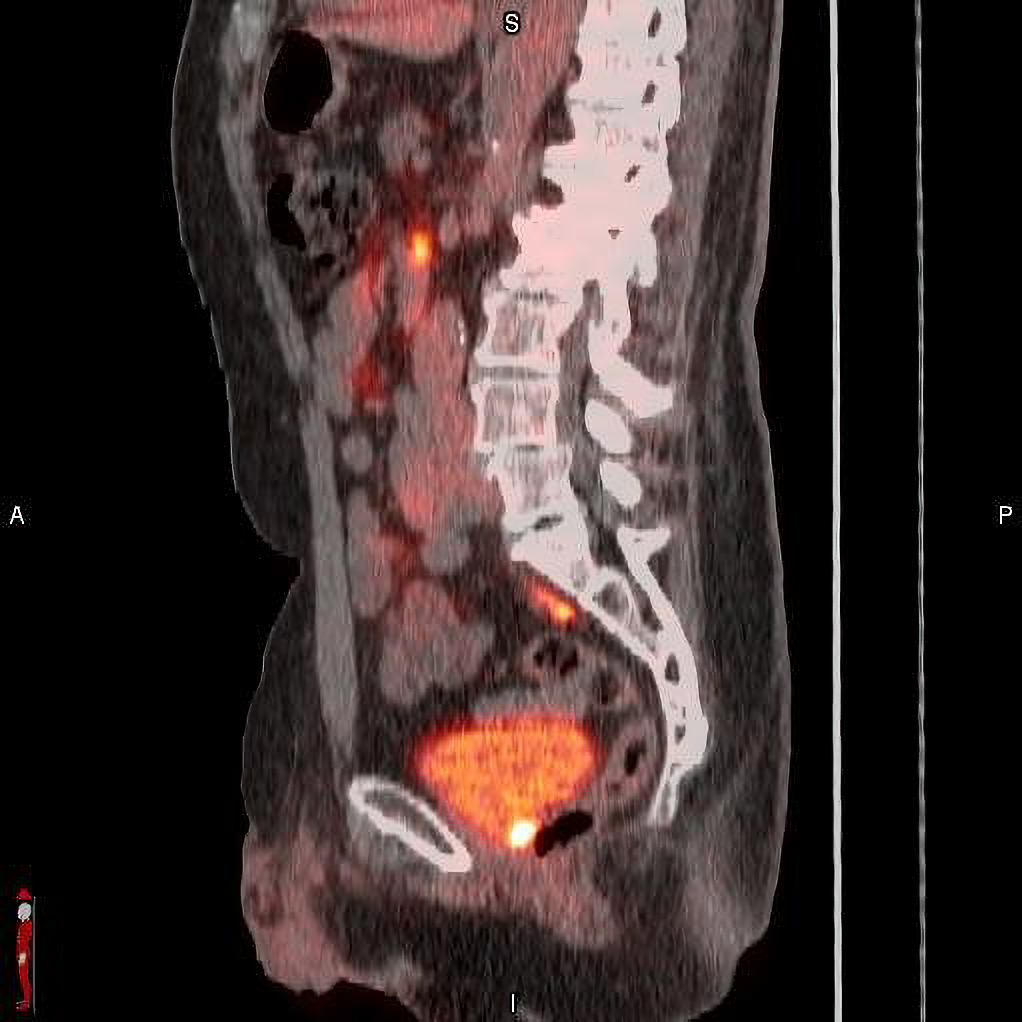
POSLUMA® (flotufolastat F 18) PET/CT image showing uptake in the prostate bed, consistent with recurrent prostate cancer. Photo courtesy of Blue Earth Diagnostics
Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative positron emission tomography (PET) radiopharmaceuticals, today announced results from a sub-analysis of data from the Phase 3 SPOTLIGHT trial (NCT04186845) which investigated the use of POSLUMA® (flotufolastat F 18) PET in suspected biochemical recurrence of prostate cancer. The sub-analysis assessed the impact of POSLUMA on treatment plans for patients with recurrent prostate cancer after curative-intent primary therapy. POSLUMA® (flotufolastat F 18) injection (formerly referred to as 18F-rhPSMA-7.3) is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
Results highlights:
“Recurrent prostate cancer presents challenges, and the ability to determine the extent and location of recurrent disease to inform appropriate clinical management is key for physicians and their patients, as up to 40% of patients who undergo radical prostatectomy, and up to 50% of patients who undergo radiation therapy will develop local or distant recurrences within 10 years,” said Przemyslaw Twardowski, MD, Saint John’s Cancer Institute at Providence Saint John’s Health Center, Santa Monica, Calif., on behalf of the SPOTLIGHT Study Group. “The SPOTLIGHT study investigated the diagnostic performance of POSLUMA PET imaging as a potential decision-making aid in assessing suspected biochemical recurrence of the disease. This sub-analysis further examined the impact of POSLUMA PET imaging on patients’ management plans. Results showed that POSLUMA identified recurrence sites in the majority of patients, frequently resulting in major changes to previously planned management plans. Patient treatment based on visualization of POSLUMA-avid lesions has the potential to facilitate optimal targeting of recurrence sites and help patients avoid futile salvage therapy.”
“We are very pleased to present these results to the oncology community at ASCO GU,” said David E. Gauden, D.Phil., Chief Executive Officer of Blue Earth Diagnostics. “POSLUMA represents a new class of high-affinity PSMA-targeted radiopharmaceuticals based on novel radiohybrid technology, and provides physicians with clinically useful information based on its performance at low PSA levels, PSMA binding and low urinary bladder activity. Our product has been added to nationally recognized clinical oncology guidelines for prostate cancer, alongside and for all the same categories as the other currently FDA-approved PSMA PET radiopharmaceuticals. POSLUMA is labeled with the radioisotope fluorine-18 (18F) to leverage high image quality and to enable broad, readily available geographic access for patients via the manufacturing and distribution network of our commercial U.S. manufacturer and distributor, PETNET Solutions Inc, A Siemens Healthineers Company.”
The findings were discussed in a moderated poster presentation at the ASCO GU 2024 Genitourinary Cancers Symposium (ASCO GU) on January 25, 2024. “Impact of 18F-flotufolastat PET on management of patients with recurrent prostate cancer: Data from the SPOTLIGHT study,” was presented by Przemyslaw Twardowski, MD, Saint John’s Cancer Institute at Providence Saint John’s Health Center, Santa Monica, Calif., on behalf of the SPOTLIGHT Study Group. Full session details are available in the ASCO GU online program here.
About the study
About Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA)