Intraarterial peptide receptor radionuclide therapy (PRRT) is a safe and effective alternative to intravenous PRRT in patients with advanced meningioma, according to a study published October 24 in the Journal of Nuclear Medicine.
Researchers at the University of Augsburg in Germany suggest the approach has the potential to deliver higher radiation doses to tumors, and found it safe in patients over a three-year follow-up period.
“[Intraarterial PRRT] may result in improved radiologic and clinical disease control compared with intravenous PRRT,” noted Adriana Amerein, MD, and colleagues.
Meningiomas are the most common primary tumors of the central nervous system, with up to 40% of cases being skull-based and ineligible for surgery because of close proximity to critical brain tissue. Intravenous PRRT with radionuclides such as Lutetium-177 (Lu-177) DOTATATE (Lutathera, Novartis) is an established second-line therapy.
In an initial case series, intraarterial administration of PRRT resulted in up to an 11-fold increase in radionuclide tumor uptake, yet these studies have been in small patient cohorts over only short follow-up periods, the authors explained. In this study, the researchers assessed both the safety and efficacy of the technique during a long-term follow-up of up to 43 months.
The group enrolled 13 patients (8 women, 5 men; mean age, 65 ± 13 years old) with advanced meningioma who subsequently underwent a median of four treatment cycles with Lu-177 DOTATATE. All patients also underwent baseline PET/CT imaging and post-treatment SPECT/CT to monitor their response.
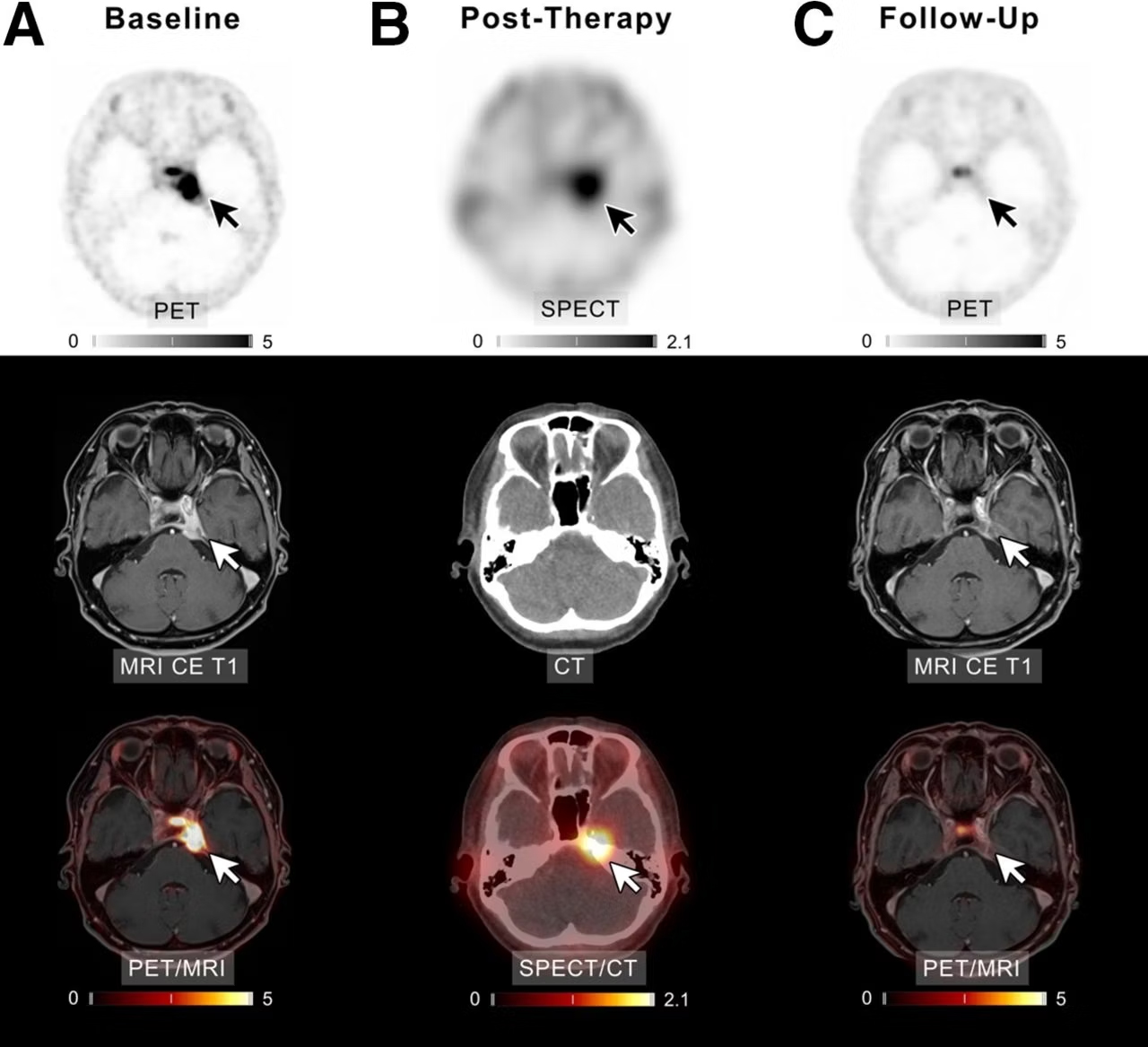
A patient with meningioma of unknown grade undergoing intraarterial PRRT with Lu-177 DOTATATE. (A) Transaxial slices of baseline PET, contrast-enhanced (CE) T1-weighted MRI, and fused PET/MRI demonstrate SSTR-expressing meningioma in left cavernous sinus (white and black arrows). After four cycles of PRRT (posttherapeutic SPECT/CT imaging after the first cycle presented in B), complete remission was recorded (C). In line with imaging, patient reported significant improvement of previous vertigo, headaches, and isolated unilateral abducens nerve palsy. Scale bars denote standard uptake values.
The treatment was generally well tolerated by all individuals, according to the findings. Immediately after PRRT or during the therapy-free interval, the most common adverse effects considered to be treatment-related were fatigue and asthenia, they wrote.
Three patients passed away one month, eight months, and 14 months after the first treatment cycle, while 10 patients showed disease control at radiologic follow-up. One of 10 had complete remission, one of 10 had partial remission, and eight of 10 had stable disease. In addition, nine patients showed good control of clinical symptoms.
“Our findings suggest that intraarterial administration of PRRT may be a therapeutic option with favorable efficacy compared with local therapies and intravenous procedures,” the group wrote.
Ultimately, the authors noted that to the best of their knowledge, the study is the first to investigate the toxicity and response to treatment with intraarterial PRRT with a long-term follow-up of up to 43 months in a population of more than 10 patients.
Future studies may explore tailoring individual doses for each patient or combining the use of external beam radiotherapy and intraarterial PRRT to further improve results for patients, the researchers suggested.
“Our study provides valuable information on the feasibility, tolerability, and efficacy of intraarterial administration of PRRT in patients with advanced progressive meningioma,” the group concluded.