An artificial intelligence (AI) model trained on whole-body scintigraphy images may be able to identify patients at risk of cardiac amyloidosis, according to a study published April 12 in JACC: Cardiovascular Imaging.
A group in France trained a deep-learning model that automatically detects cardiac radiotracer uptake on technetium-99m (Tc-99m) whole-body scintigraphy from a large hospital dataset. The model performed well and could be used perhaps to help identify thousands of undiagnosed patients every year, wrote lead author Dr. Marc-Antoine Delbarre, of Amiens University Hospital, and colleagues.
"The use of our automatic cardiac fixation detection model in nuclear medicine centers will probably help to find undiagnosed cardiac amyloidosis in the hundreds of thousands of patients who have undergone or are undergoing [whole-body scintigraphy]," the group wrote.
Cardiac amyloidosis is a group of protein misfolding disorders caused by progressive deposits of amyloid fibrils in the myocardium and can lead to death by heart failure. Because of nonspecific cardiac symptoms, patients often suffer from a delayed diagnosis.
Bone scintigraphy is typically performed to diagnose bone tumors, yet uptake of the technetium-99m (Tc-99m) radiotracer used in the scans by other regions in the body may indicate pathology associated with other disease. This is the case in cardiac amyloidosis, the researchers explained.
In this study, the authors hypothesized that a deep-learning model could be developed that searches for significant cardiac radiotracer uptake in a dataset of Tc-99m whole-body scintigraphy images to help identify undiagnosed patients.
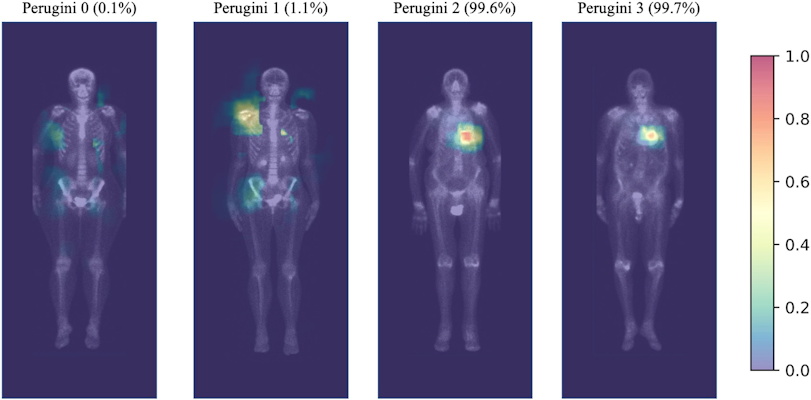
Highlighted areas contribute the most to the positive prediction in the convolution layers. For Perugini 2, 3, and false positive images, the heart area is clearly highlighted. The predicted probability of each image to be positive is indicated at the top of the image. On the right is the probability scale. Image and caption courtesy of JACC: Cardiovascular Imaging through CC BY 4.0.
The group trained a deep-learning model to identify the disease based on myocardium tracer uptake using a standard Perugini grading system, which is based on a visual score of the image from grade 0 (negative) to 3 (strongly positive). The training dataset consisted of 3,048 images, 281 positive (≥ Perugini 2) and 2,767 negative cases from Henri Mondor University Hospital in Creteil.
The investigators then tested the model on an external dataset culled from Lille University Hospital consisting of 1,633 images, 102 of which were positive and 1,531 of which were negative.
According to the analysis, across both the datasets, the model's performance remained constant, with an accuracy of 99.3% and a specificity of 99.5%, the authors reported.
| Average rate of AI-predicted positive cases by Perugini grade | ||
| Grade | Henri Mondor | Lille |
| Grade 0 | 1.1% | 0.3% |
| Grade 1 | 8.4% | 16% |
| Grade 2 | 96.4% | 92% |
| Grade 3 | 100% | 100% |
"The ... detection model is effective at identifying patients with cardiac uptake [equal to or more than] Perugini 2 on whole-body scintigraphy and may help in the diagnosis of patients with cardiac amyloidosis," the group wrote.
Yet further research is warranted, the authors noted, especially given that this is the first model to suggest AI may be efficient at detecting uptake of Perugini grade equal to or greater than 2 for cardiac amyloidosis. They also wrote that Tc-99m radiotracer uptake in the myocardium may reveal other conditions.
"The detection of significant cardiac uptake may lead to the diagnosis of [cardiac amyloidosis] only after rigorous evaluation according to current recommendations in order to distinguish amyloidosis-related from non-amyloidosis-related uptake," the group concluded.