The 2025 American Society of Clinical Oncology (ASCO) Annual Meeting held in Chicago, IL, was host to the Poster Session: Genitourinary Cancer - Prostate, Testicular, and Penile Cancer. Dr. Jeremie Calais presented the trial in progress Poster TPS5110: Phase 2 trial of re-treatment with 177Lu-PSMA-617 molecular radiotherapy for metastatic castration resistant prostate cancer (RE-LuPSMA trial)
The Phase III VISION trial established 177Lu-PSMA-617 radioligand therapy as a life-prolonging treatment for patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with taxane-based chemotherapy and at least one androgen receptor pathway inhibitor (ARPI).1 Based on these results, the U.S. FDA approved 177Lu-PSMA-617 for up to six cycles (7.4 GBq every 6 weeks) in this population.
Despite initial responses, 177Lu-PSMA-617 is not curative, and most patients eventually experience disease progression. At that point, therapeutic options are limited, especially for those who have already received both chemotherapy and ARPI-based treatments.
Re-treatment with 177Lu-PSMA-617 in patients who previously responded and experienced manageable toxicity has emerged as a potential strategy. Several small retrospective studies have reported encouraging outcomes with this approach. However, prospective data with larger patient cohorts are needed to validate these findings and better define the role of re-administration in the treatment landscape of mCRPC. Dr Calais and colleagues aim to evaluate the efficacy and safety of re-challenge therapy using 177Lu-PSMA-617 in patients who responded well to a previous regimen of 177Lu-PSMA-617.
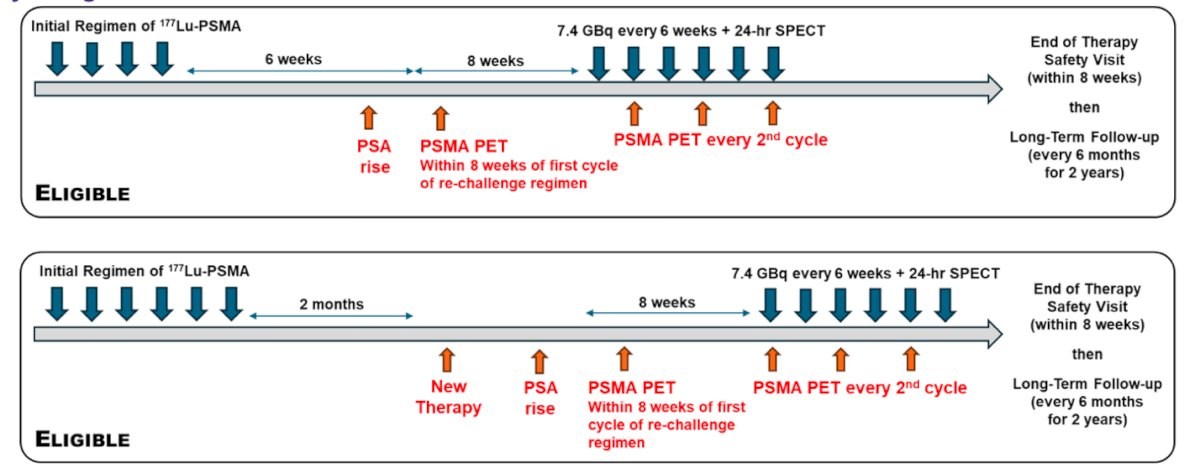
RE-LuPSMA is an investigator-initiated, single-arm, single-center, open-label Phase 2 clinical trial (NCT06288113) evaluating the efficacy and safety of re-treatment with ^177Lu-PSMA-617 in men with mCRPC. The trial aims to enroll 40 patients, with accrual expected within the first 12 months of a planned 4-year study duration. Enrollment is currently ongoing.
The inclusion criteria are summarized below:

Dr. Calais highlighted that patients enrolled in the RE-LuPSMA trial will receive up to six cycles of 177Lu-PSMA-617 re-challenge therapy, administered at a dose of 7.4 GBq every six weeks (±1 week).
To monitor treatment response and biodistribution:
The study design is illustrated below:
Primary Endpoint:
Key Secondary Endpoints:
Presented by: Jeremie Calais, MD, PhD, Ahmanson Translational Theranostics Division, University of California, Los Angeles, CA.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the American Society of Clinical Oncology (ASCO) 2025 Annual Meeting, Chicago, IL, Fri, May 30 – Tues, Jun 3, 2025.
Reference: